Describe the Relationship Between Moles and Atoms
Water H2O is a molecule formed by the combination of two different atoms- hydrogen H and oxygen O in a fixed 21. One mole contains 602 10.
2 8 Atoms And The Mole How Many Particles Chemistry Libretexts
A sample of 2 tsp of sugar C12H220 weighs 900 g a.

. 1 mole 60221023 6022 10 23 atoms molecules protons etc. One mole of atoms of an element has a mass equivalent to the mass number now sometimes called nucleon number of that element eg. View the full answer.
Mass of element m n M. Summarize fill in the blanks to help you take. A sample of 2 tsp of sugar C12H22011 weighs 900 g.
Describe the relationship between moles and atoms. Mole is reserved for measuring small units like atoms or molecules. Course Title CHE 101L.
Exercise-1 C Using the data table-1. Summary of Atoms vs. Then calculate the moles and atoms of each element in the sample of sugar.
Find the Molecular mass of all atoms in the chemical formula and divide it with the mass of the sunstance to get the amount of moles aariyahjc9874 aariyahjc9874 02082020 Chemistry High School answered Describe the relationship between the mole information of a substance and its chemical formula. How is the atomic mass of an element related to the molar mass of the element. 602 1023 representative particles ____.
3 O atom in cotton ball C 6 H 10 O 5. Molar mass M. 602x1023 atoms 1 mol of carbon b.
Describe the relationship between moles and atoms. B US ET. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal.
3 il 111 т T o Word s 2. The total number of atoms of each element must be the same on each side of the equation to satisfy the Law of _____. This preview shows page 1 -.
Every substance is actually a huge amount particles atoms or molecules. For example O2 is a molecule formed by the combination of two oxygen atoms. Record each step needed to calculate the moles and atoms of all elements present in the sample.
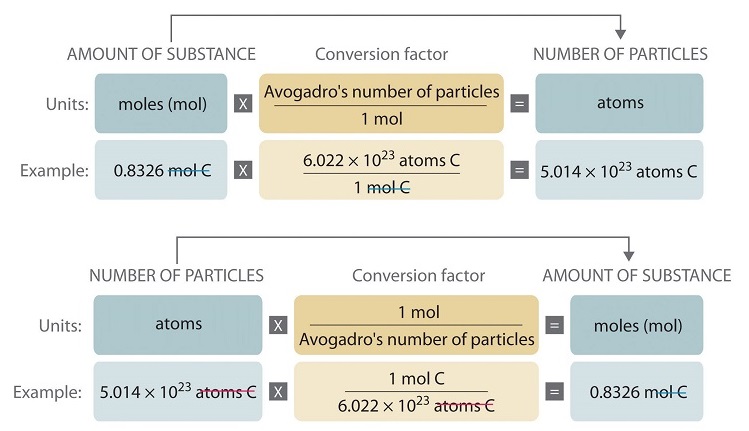
School Eastern Kentucky University. Describe the relationship between moles and atoms. To convert from moles to atoms multiply the molar amount by Avogadros number.
Read formulas definitions laws from Relation Between Mole mass and Number of Atoms here. Click here to learn the concepts of Relation Between. Ratings 88 8 7 out of 8 people found this document helpful.
Given number of particles N. 50 g which sample wo. Experts are tested by Chegg as specialists in their subject area.
Are not equal to whole numbers what must you do. Describe the relationship between moles and atoms. Avogadro number of particles N 0.
One mole of any substance contains Avogadro Nu. 602x1023 atoms 1 mol of oxygen. 301 x 10 22 atom.
When determining a compounds empirical formula if your calculated mole amounts are not equal to whole numbers you round up or down. Mole is an unit for the fundamental physical quantity called amount of substance. State the mathematical relationship between Avogadros number and 1 mol.
Answer 1 of 2. Record each step needed to calculate the moles and atoms of all Q. 1 mole of _612C has a mass of 12 g.
A calculation of the _____ _____ of a reactant or product enables us to convert from grams of a particular substance taking part in a reaction to moles of that substance. Technically its slightly more complex than the answer above. Exercise 1 1 describe the relationship between moles.
See the answer See the answer done loading. Record each step needed to calculate the moles and atoms of all elements present in the sample. These relations can be interchanged as.
A sample of 2 tsp of sugar C H O weighs 900 g. Who are the experts. Experts are tested by Chegg as specialists in their subject area.
Avogadros number is a very important relationship to remember. Record each step needed to calculate the moles and atoms of all elements present in the sample. Allows us to express atomic mass in terms of grams To convert from moles to atoms multiply the molar amount by Avogadros number.
We review their content and use your feedback to keep the quality high. List the conversion factors used to convert between particles and moles. A Describe the relationship between moles and atoms 2.
We review their content and use your feedback to keep the quality high. We can have a mole of atoms molecules ions. Experts are tested by Chegg as specialists in their subject area.
Show all work to answer this question by uploading an image. A mole is simply a quantity of 6022xx1023 of anything. Amount of oxygen atom in each item are followed as 1 O atom in aluminium cup Al 2 O 3.
This diagram explains the relationship between atomsmoles and molecules. In a nutshell the bridge between atoms and moles is the Avogadros number which is 602210 23. Avogadros number is the basis for the mole as it makes calculating the mass or weight of one mole quite easy.
Moles molecules atoms. Then calculate the moles and atoms of each element in the sample of sugar. The mole relationship given by.
Of particles of element N n N 0. Amount of substance as a physical quantity tells us how many particles are there in some amount of some substance. Tz O Word s 2.
Describe the relationship between Avogadros number and a mole of a substance. 190 x 10 23 atom. Now from these three data it clear that the cotton ball has the least.
100 11 ratings 1 mole is defined as the amount of an atom or ion or molecule or compound that contains. We review their content and use your feedback to keep the quality high. Describe the relationship between Avogadros number and one mole of any substance.
Then calculate the moles and atoms of each element in the sample of sugar. A mole of any substance always contains Avogardos number of represenative particles or 602 x 1023 particles. Chemists use the mole because it is a convenient way of knowing how many representative particles are in a sample.
A sample of 2 tsp of sugar C12H22O11 weighs 900 g. Similarly 1 Mole of molecules 6022 10 23 molecules. Describe the relationship between moles and atoms.
If you were given a sample of a cotton ball and a glass stirring rod with identical mass ex. 433 x 10 22 atom. 190 x 10 23 433 x 10 22 301 x 10 22.
Who are the experts. A mole is a number used to convert a certain arbitrary number of atoms into grams. Chemistry questions and answers.
Gram molecular mass of Molar mass. 2 O atom in glass stirring rod SiO 2. Describe the relationship between moles and atoms.
Difference Between Atom And Mole Difference Between

Moles To Atoms Conversion Chemistry Youtube

What Is The Relationship Between A Mole And Avogadro S Number A Plus Topper

What Is The Relationship Between A Mole And Avogadro S Number A Plus Topper
No comments for "Describe the Relationship Between Moles and Atoms"
Post a Comment